 |
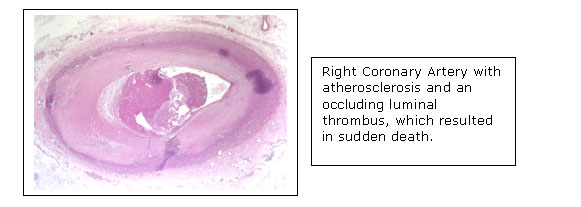
Projects Atherosclerosis is the principal cause of heart disease and a leading cause of stroke, making it the most common cause of death in the U.S. The laboratory is seeking to understand the biochemical processes resulting in atherosclerosis in order to combat this pervasive disease.
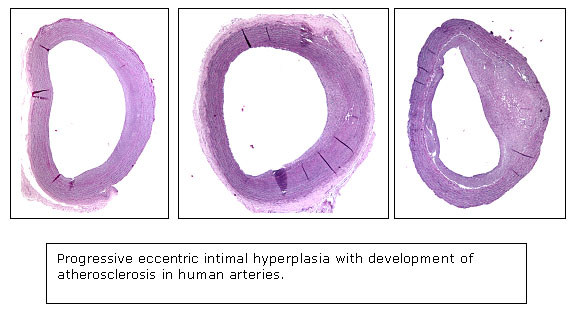
Atherosclerosis is characterized by the development of necrotic/lipid cores within the intima of arteries at particular sites in the circulation. These necrotic/lipid cores form in the setting of a pre-existing intimal hyperplasia, characterized by the proliferation of smooth muscle-like cells within the intima. The laboratory is investigating both the mechanisms of signal transduction responsible for the formation of the pre-atherosclerotic intimal hyperplasia, as well as the factors stimulating the formation of intimal necrotic/lipid cores.
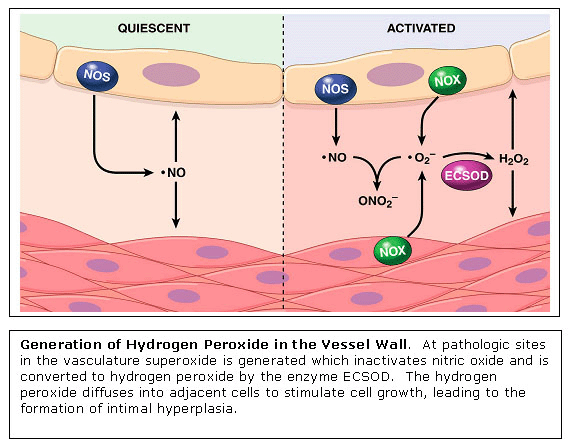
Signal Transduction with Hydrogen Peroxide in Vascular Cells Essentially
all risk factors for atherosclerosis result in the enhanced generation
of hydrogen peroxide
The
mechanisms by which low endogenous levels of hydrogen peroxide stimulate
cellular proliferation are currently poorly understood. The laboratory
is using proteomic approaches with cultured vascular cells to identify
signal transduction pathways activated by low physiologic levels of
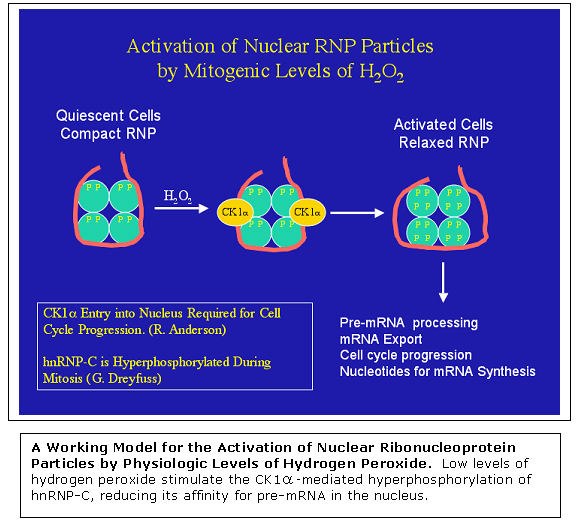
hydrogen peroxide. One target protein identified is the nuclear pre-mRNA
binding protein hnRNP-C. Low physiologic levels of hydrogen peroxide
stimulate the hyperphosphorylation of the acidic C-terminal domain
of hnRNP-C, resulting in diminished ability of the protein to bind
mRNA. The effect is mediate by protein kinase CK1
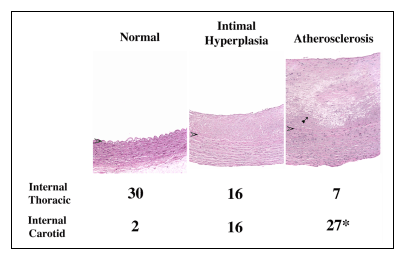
Proteomic Analyses of Intimal Hyperplasia from Atherosclerosis-Prone and Atherosclerosis-Resistant Human Arteries Pre-atherosclerotic
intimal hyperplasia forms at branch sites both in arteries prone to
develop atherosclerosis, such as the internal carotid and coronary
arteries and also in vessels remarkably resistant to the formation
of atherosclerosis, such as the internal thoracic artery and the distal
ulnar artery. The structural variations in intimal hyperplasia that
may facilitate the development of atherosclerosis have been unclear.
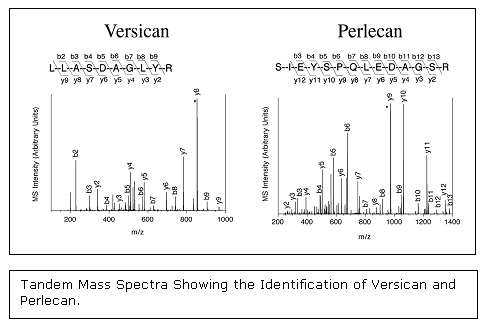
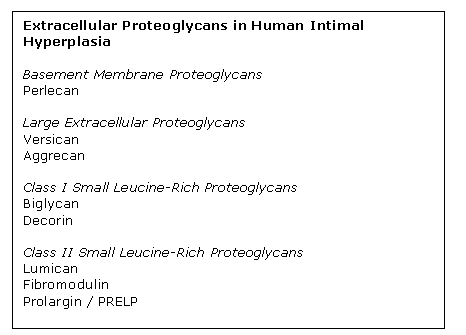
Proteoglycans have been implicated as playing a direct role in atherosclerosis, both by binding and retaining lipoproteins in the vessel wall and by regulating cell growth. One project in the laboratory has been to analyze by mass spectrometry the extracellular proteoglycans present in pre-atherosclerotic intimal hyperplasia from atherosclerosis-prone arteries as well as atherosclerosis-resistant arteries. This project has revealed the proteoglycan composition of human intimal hyperplasia to be more complex than previously realized with eight distinct proteoglycans present: perlecan, versican, aggrecan, biglycan, decorin, fibromodulin, lumican, and prolargin.
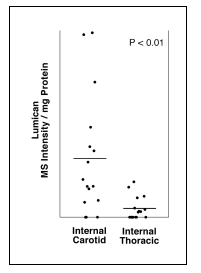
Importantly, while most of the proteoglycans are present at similar levels in the two arterial types, there is a selective enhanced deposition of lumican proteoglycan in the pre-atherosclerotic intimal hyperplasia from the atherosclerosis-prone artery compared with the intimal hyperplasia from the atherosclerosis-resistant artery.
This data suggests that lumican may play a central role in the development of atherosclerotic lesions in humans, and may partly account for site-specific susceptibility to atherosclerosis. |
|
©2012 James R. Stone Lab - Department of Pathology - Massachusetts General Hospital |